Reverse osmosis is a method of filtration, based on the physical phenomenon called osmosis. The main propelling force of the process is pressure – the difference between pressures before and after the membrane leads to transporting the substance through the membrane. The principle of the process lies in water molecules from salt solutions being forced by pressure to permeate through the semi-permeable membrane, which leads to a concentrated solution of salts on the pressure side of the membranes and clear solvent (water) on the other side. An ideal reverse osmosis membrane only lets through water, while hydrated ions and low-molecular substances are stopped by the membrane. For passing of water through the membrane, it is necessary to overcome osmotic pressure which can be in the order of tens of bars in case of grossly salinized water.
Reverse osmosis is the most common pressure membrane process. The first application connected with the utilization of reverse osmosis date back to 1970s. At that time, reverse osmosis membranes were produced from cellulose acetate, the scope of their use was very limited, and generally, reverse osmosis was not considered an economically profitable process, mainly due to high operation costs. In 1980s, the rapid development of new types of reverse osmosis membranes began. These long-life membranes were more resistant to high pressure, pH fluctuation and biological fouling. At present, composite membranes are used, made of several layers of various polymers. Utilized modules are almost exclusively spirally wounds, exceptionally of hollow fibres.
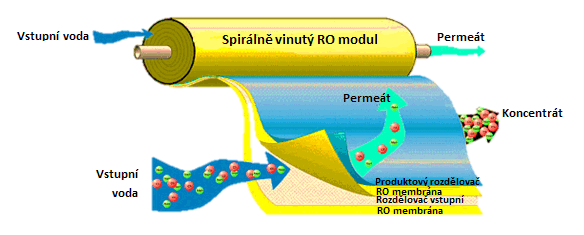
The process of reverse osmosis is used for desalination of sea and brackish water, in production of purified water for electrical engineering and pharmaceutical industry and in purification of some types of industrial waste water. Reverse osmosis is also applied in some branches of food processing, chemical and power engineering industries.



